Our Research
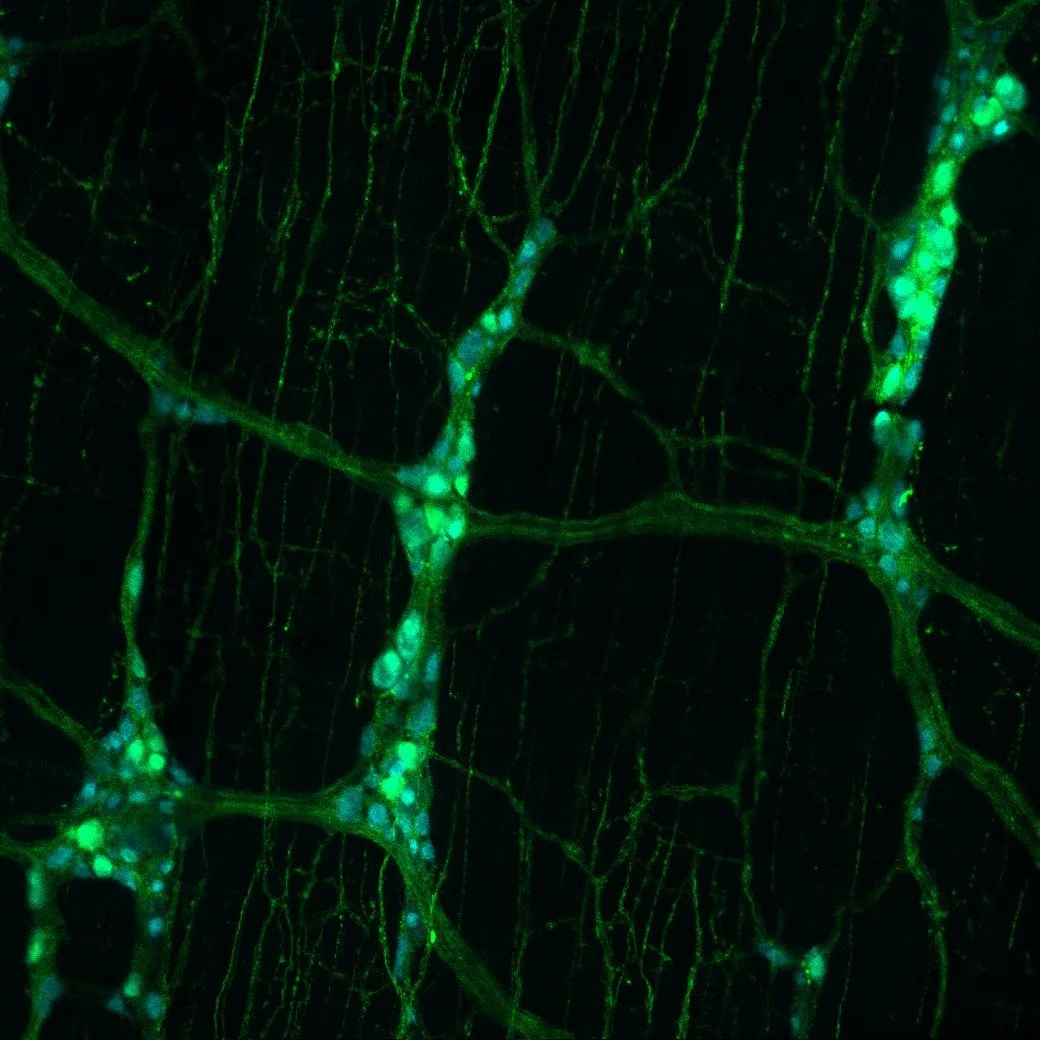
A Plaza in Texas in the 1930’s by Carmen Lomas GarzaEnteric Circuits
Like the gathered groups of people in A Plaza in Texas in the 1930s, shown in Carmen Lomas Garza’s vibrant painting, the gut contains scattered but organized clusters of neurons and glia. These clusters span its entire length, from the esophagus to the colon. Together, these clusters make up the enteric nervous system (ENS), an intrinsic neural network that controls gut reflexes such as motility, secretion, and absorption. Despite its central role in gut physiology, much remains unknown about how ENS circuits are wired and regulated, how they interact with the sensory and autonomic nervous systems, and how they function in vivo. New genetic tools in mice now make it possible to address these gaps. By combining molecular genetics, high-resolution imaging, and in vivo approaches, we aim to map enteric circuits, uncover regulatory mechanisms, and define how ENS dysfunction disrupts gut function.
Allegory of Winter (1948) by Remedios Varo
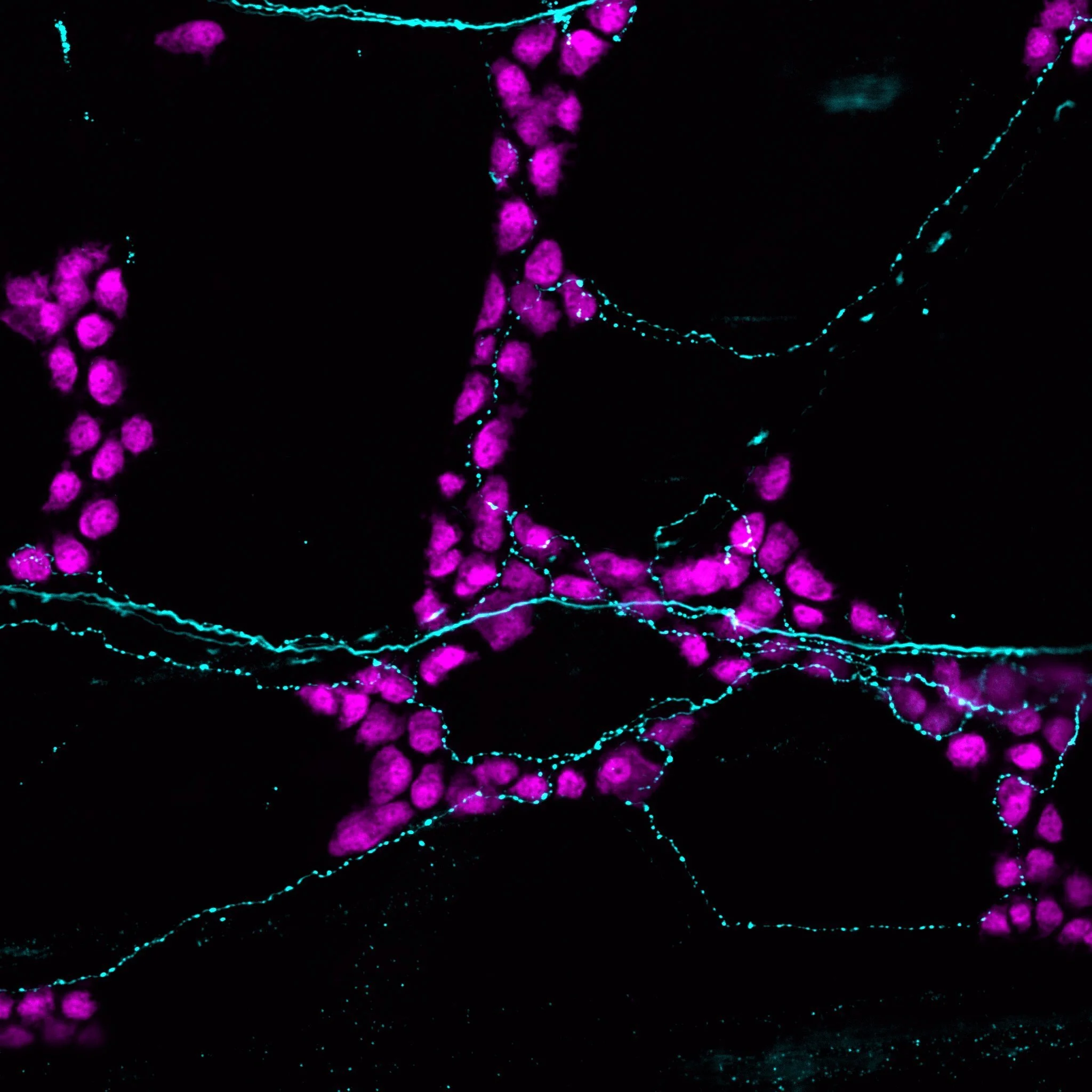
In Remedios Varo’s Allegory of Winter, branching trees extend toward translucent geometric forms that hold encapsulated elements, evoking hidden connections between two worlds. We draw a similar parallel to the gut, where sensory neurons innervate muscle, epithelial, immune, and enteric neurons. In other sensory systems, such as the skin, these neurons form specialized complexes with epithelial cells to mediate touch. Whether comparable structural or functional relationships exist in the gut remains unknown. Our research aims to determine whether sensory neurons form such connections with enteric neurons and other gut-resident cells, and to explore their potential roles in maintaining gut health.
Sensory Neuron–Gut Cell Interactions
Without Hope by Frida Kahlo, 1945
In Frida Kahlo’s Without Hope, a funnel delivers fragments of flesh, fish, and meat into the artist’s mouth. We interpret this image as a metaphor for how overwhelming stressors can disrupt the gut–brain axis and unsettle its balance. In our lab, we study factors such as disease, psychological stress, and unbalanced diets to understand how they alter gut–brain communication. We aim to define the molecules, neuronal subtypes, and circuits involved, and ultimately the mechanisms that drive their effects. By uncovering these processes, we seek to distinguish the pathways that sustain gut health from those that drive symptoms such as dysbiosis, inflammation, and dysmotility, which impair quality of life.